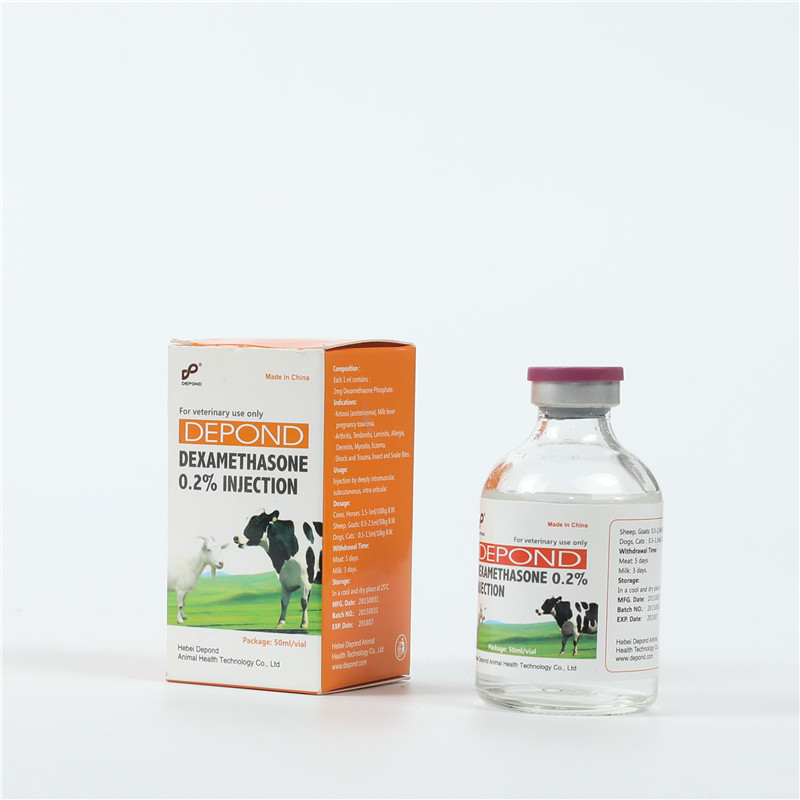
OEM/ODM Manufacturer Dexamethasone Injectable Solution - Dexamethasone Injection – Depond
OEM/ODM Manufacturer Dexamethasone Injectable Solution - Dexamethasone Injection – Depond Detail:
Composition
Each ml contains:
Dexamethasone sodium phosphate 2 mg.
Excipients up to 1 ml.
Descriptions
Colorless clear liquid.
Pharmacological action
The drug exerts it`s pharmacological action by penetrating and binding to cytoplasmic receptor protein and causes a structural change in steroid receptor complex. This structural change allows it`s migration in to the nucleus and then binding to specific sites on the DNA which leads to transcription of specific m-RNA and which ultimately regulates protein synthesis. It exerts highly selective glucocorticoid action. It stimulates the enzymes needed to decrease the inflammatory response.
Indications
Metabolic disorders, non-infectious inflammatory processes, especially acute musculoskeletal inflammations, allergic conditions, stress and shock conditions. As an aid in infectious diseases. Induction of parturition in ruminants during the last stage of pregnancy.
Dosage and administration
For intravenous or intramuscular injection.
Cattle : 5-20mg (2.5-10ml) per time.
Horses: 2.5-5mg (1.25-2.5ml) per time.
Cats : 0.125-0.5mg (0.0625-0.25ml) per time.
Dogs: 0.25-1mg (0.125-0.5ml) per time.
Side effect and contraindication
Except for emergency therapy, do not use in animals with chronic nephritis and hyper-corticalism (Cushing’s Syndrome). Existence of congestive heart failure, diabetes, and osteoporosis are relative contraindications. Do not use in viral infections during the viremic stage.
Caution
Care should be taken to avoid accidental self-injection.
Once vial has been broached, contents must be used within 28 days.
Dispose of any unused product and empty containers.
Wash hand after use.
Withdrawal Period
Meat: 21 days.
Milk: 72 hours.
Storage
Store in a cool and dry place below 30℃.
Product detail pictures:


Related Product Guide:
We pursue the management tenet of "Quality is remarkable, Company is supreme, Name is first", and will sincerely create and share success with all clientele for OEM/ODM Manufacturer Dexamethasone Injectable Solution - Dexamethasone Injection – Depond , The product will supply to all over the world, such as: Botswana, Auckland, Tunisia, High output volume, top quality, timely delivery and your satisfaction are guaranteed. We welcome all inquiries and comments. We also offer agency service---that act as the agent in china for our customers. If you are interested in any of our products or have an OEM order to fulfill, please feel free to contact us now. Working with us will save you money and time.
The product manager is a very hot and professional person, we have a pleasant conversation, and finally we reached a consensus agreement.